Exploring Bispecific Antibody Development
The Evolution of Bispecific Antibody Development
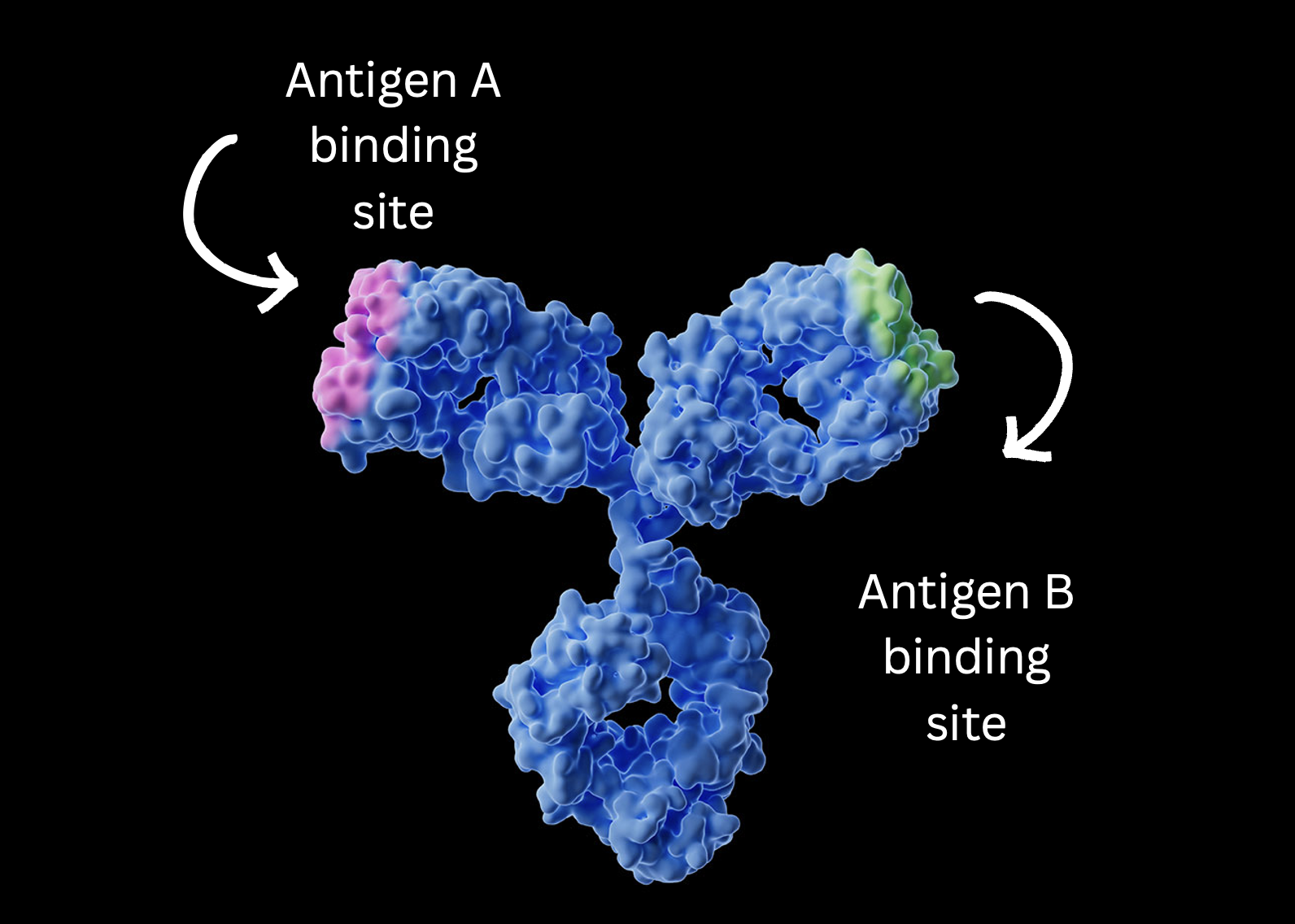
Bispecific antibodies represent a significant advancement in therapeutic antibody development. Unlike traditional monoclonal antibodies, which target a single antigen, bispecific antibodies are engineered to recognize and bind to two different antigens simultaneously. This dual targeting capability enables more precise intervention in complex biological processes, making bispecific antibodies a powerful tool in treating diseases such as cancer and autoimmune disorders.
The development of bispecific antibodies involves sophisticated engineering techniques to ensure stability, efficacy, and safety. Early designs faced challenges related to stability and manufacturability, but advancements in protein engineering have led to the creation of more robust and effective bispecific constructs. These innovations allow for greater flexibility in designing antibodies that can target specific cellular pathways or immune checkpoints.
Bispecific antibody development is driven by the need for more effective treatments that can overcome resistance mechanisms often encountered with traditional therapies. By targeting multiple pathways or combining immune activation with direct tumor targeting, bispecific antibodies offer a promising approach to improving patient outcomes.
Platforms and Technologies in Bispecific Antibody Production
The production of bispecific antibodies relies on various platforms and technologies designed to optimize their function and manufacturability. Several approaches are used in the design and production of bispecific antibodies, including the use of linker technologies, tandem single-chain variable fragments (scFvs), and dual-variable domain immunoglobulins.
Linker technologies are essential for connecting the two antigen-binding sites while maintaining their stability and functionality. These linkers must be carefully designed to ensure that the bispecific antibody retains its ability to bind both targets simultaneously without compromising its structural integrity.
Tandem scFvs involve the fusion of two single-chain antibody fragments into a single molecule, allowing for dual targeting capabilities. This approach simplifies the production process while ensuring high specificity and affinity for the chosen antigens.
Dual-variable domain immunoglobulins consist of two variable domains derived from different antibodies, engineered into a single IgG-like structure. This format closely resembles natural antibodies, providing stability and ease of production while enabling bispecific targeting.
T Cell Bispecific Antibodies: A New Era of Immunotherapy
Harnessing T Cell Activation with Bispecific Antibodies
T cell bispecific antibodies have emerged as a groundbreaking approach in immunotherapy, leveraging the body's immune system to target and destroy cancer cells. These antibodies are designed to bind simultaneously to a target antigen on cancer cells and a CD3 molecule on T cells, effectively recruiting T cells to the tumor site and activating them to exert their cytotoxic effects.
The ability to directly engage T cells offers several advantages over traditional cancer treatments. By harnessing the body's natural defense mechanisms, T cell bispecific antibodies can enhance immune responses, reduce tumor burden, and potentially lead to long-lasting remission. This approach minimizes the need for exogenous immune stimulants, reducing the risk of severe side effects commonly associated with traditional chemotherapy or radiation therapy.
T cell bispecific antibodies are particularly effective in overcoming immune evasion strategies employed by tumors. By facilitating direct interactions between T cells and cancer cells, these antibodies can bypass inhibitory signals and enhance the immune system's ability to recognize and eliminate malignant cells.
Challenges and Opportunities in T Cell Bispecific Antibody Development
While T cell bispecific antibodies offer promising therapeutic potential, their development presents several challenges. Ensuring specificity and minimizing off-target effects are critical to reducing the risk of unintended immune activation and associated toxicities. Precise engineering is required to balance potency with safety, ensuring that T cell bispecific antibodies selectively target tumor cells while sparing healthy tissues.
Manufacturing T cell bispecific antibodies at scale also poses challenges due to their complex structure and binding requirements. Advances in protein engineering, purification methods, and scalable production technologies are essential to overcoming these hurdles and bringing these therapies to broader patient populations.
Despite these challenges, the opportunities for T cell bispecific antibodies in cancer treatment are immense. Ongoing research and clinical trials continue to explore their efficacy across various cancer types, offering hope for patients who have exhausted other treatment options. As technology advances, T cell bispecific antibodies are expected to play a central role in the next generation of immunotherapies.
Dual Targeting Strategies with Bispecific Antibodies
The Power of Dual Targeting in Disease Intervention
Dual targeting strategies with bispecific antibodies revolutionize therapeutic approaches by enabling simultaneous modulation of multiple biological pathways. This capability is particularly advantageous in diseases characterized by complex pathophysiology, such as cancer, where multiple signaling pathways contribute to tumor growth and progression.
By targeting two distinct antigens, bispecific antibodies can disrupt critical interactions that drive disease processes. For example, in cancer therapy, bispecific antibodies may simultaneously inhibit tumor growth signals and enhance immune cell recruitment to the tumor microenvironment. This dual action not only increases therapeutic efficacy but also reduces the likelihood of resistance development.
Dual targeting also offers opportunities for personalized medicine, allowing for the customization of treatment regimens based on individual patient profiles. By integrating genomic and proteomic data, bispecific antibodies can be tailored to target specific molecular signatures present in a patient's tumor, optimizing treatment outcomes.
Innovative Applications of Dual Targeting Strategies
The versatility of dual targeting strategies extends beyond oncology, with potential applications in autoimmune diseases, infectious diseases, and neurological disorders. In autoimmune conditions, bispecific antibodies can simultaneously modulate immune checkpoints and inflammatory pathways, restoring immune balance and preventing tissue damage.
In infectious diseases, bispecific antibodies can target viral antigens and immune effector cells, enhancing viral clearance and reducing disease severity. Their ability to engage multiple components of the immune system offers a promising approach to combating emerging viral threats.
Neurodegenerative disorders, characterized by complex interactions between genetic, environmental, and immune factors, can also benefit from dual targeting strategies. Bispecific antibodies designed to target specific protein aggregates and modulate neuroinflammation offer a novel approach to slowing disease progression and preserving cognitive function.
CAR T vs. Bispecific Antibodies: Comparative Insights
Understanding CAR T Cell Therapy
CAR T cell therapy involves genetically modifying a patient's T cells to express chimeric antigen receptors (CARs) that recognize and attack cancer cells. This personalized approach has shown remarkable success in treating certain hematological malignancies, leading to durable remissions in patients with advanced disease.
CAR T cells are engineered to target specific antigens present on cancer cells, providing highly selective and potent immune responses. The ability to expand and persist within the body allows CAR T cells to maintain long-term antitumor activity, offering hope for curative outcomes in some cases.
Comparing CAR T and Bispecific Antibody Therapies
While both CAR T cell therapy and bispecific antibodies aim to harness the immune system to combat cancer, they differ in their mechanisms and applications. CAR T therapy involves cellular engineering and requires individualized manufacturing, making it time-consuming and expensive. However, its success in specific blood cancers highlights its potential for personalized treatment.
Bispecific antibodies, on the other hand, offer broader applicability across various cancer types and do not require complex cellular manipulation. Their dual targeting capabilities allow for immediate therapeutic effects upon administration, potentially reducing the time to clinical response.
The choice between CAR T and bispecific antibody therapies depends on factors such as cancer type, patient condition, and treatment goals. While CAR T therapy excels in personalized approaches for certain hematological cancers, bispecific antibodies offer versatility and scalability for a wider range of indications.
Integrating CAR T and Bispecific Antibody Strategies
As the field of immunotherapy advances, integrating CAR T and bispecific antibody strategies could enhance treatment outcomes. Combining these approaches may offer synergistic effects, leveraging the strengths of each modality to overcome limitations and improve patient responses.
Research exploring combination therapies is ongoing, with the potential to expand the range of treatable cancers and optimize therapeutic regimens. By harnessing the power of both cellular and antibody-based interventions, the future of cancer treatment holds promise for more effective and comprehensive solutions.
The Future of Bispecific Antibody Platforms
Advancements in Bispecific Antibody Platforms
Bispecific antibody platforms are at the forefront of therapeutic innovation, offering flexible designs and scalable production techniques. The development of novel platforms aims to optimize bispecific antibody function, manufacturability, and accessibility for various clinical applications.
Advancements in platform technologies focus on improving target specificity, reducing immunogenicity, and enhancing pharmacokinetics. Innovations in protein engineering and computational modeling enable the design of bispecific antibodies with improved stability and reduced off-target effects.
Scalable production methods, including recombinant DNA technology and advanced purification techniques, ensure that bispecific antibodies can be manufactured efficiently and cost-effectively. These advancements are crucial for bringing bispecific therapies to market and making them accessible to patients worldwide.
The Role of Bispecific Antibody Platforms in Personalized Medicine
Bispecific antibody platforms play a pivotal role in advancing personalized medicine by enabling the customization of therapies based on individual patient profiles. By integrating genomic and proteomic data, bispecific antibodies can be tailored to target specific molecular signatures present in a patient's disease, optimizing treatment efficacy.
The flexibility of bispecific platforms allows for the rapid adaptation of therapies to emerging disease targets and resistance mechanisms. As precision medicine continues to evolve, bispecific antibodies are expected to play a central role in developing targeted therapies that address the unique needs of each patient.
FAQ:
What are bispecific antibodies?
Bispecific antibodies are engineered proteins designed to bind two different antigens simultaneously, offering dual targeting capabilities for enhanced therapeutic efficacy.
How do bispecific antibodies differ from CAR T cell therapy?
Bispecific antibodies target multiple antigens directly, without cellular manipulation, while CAR T therapy involves engineering T cells to express specific receptors that target cancer cells.
What diseases can bispecific antibodies treat?
Bispecific antibodies are used in cancer, autoimmune diseases, infectious diseases, and neurological disorders, offering versatile applications across various indications.
What are the challenges in developing bispecific antibodies?
Challenges include ensuring specificity, minimizing off-target effects, and optimizing manufacturing processes for scalable production.
How do bispecific antibody platforms contribute to personalized medicine?
Bispecific platforms enable the customization of therapies based on individual molecular profiles, enhancing treatment specificity and efficacy.